iClinical Inc
iClinical is a real-time analytics and collaboration platform for clinical trials.
We help biotech and pharma companies bring life saving drugs to the market sooner and a lower cost.
The problem
Clinical trial technology is broken. Use of paper to collect data. Data is several disparate servers and finally, data is not analyzed in real-time. Several plug-in solutions/vendors are used to complete clinical trials. These obselete technology/processes add time and cost to clinical trials.
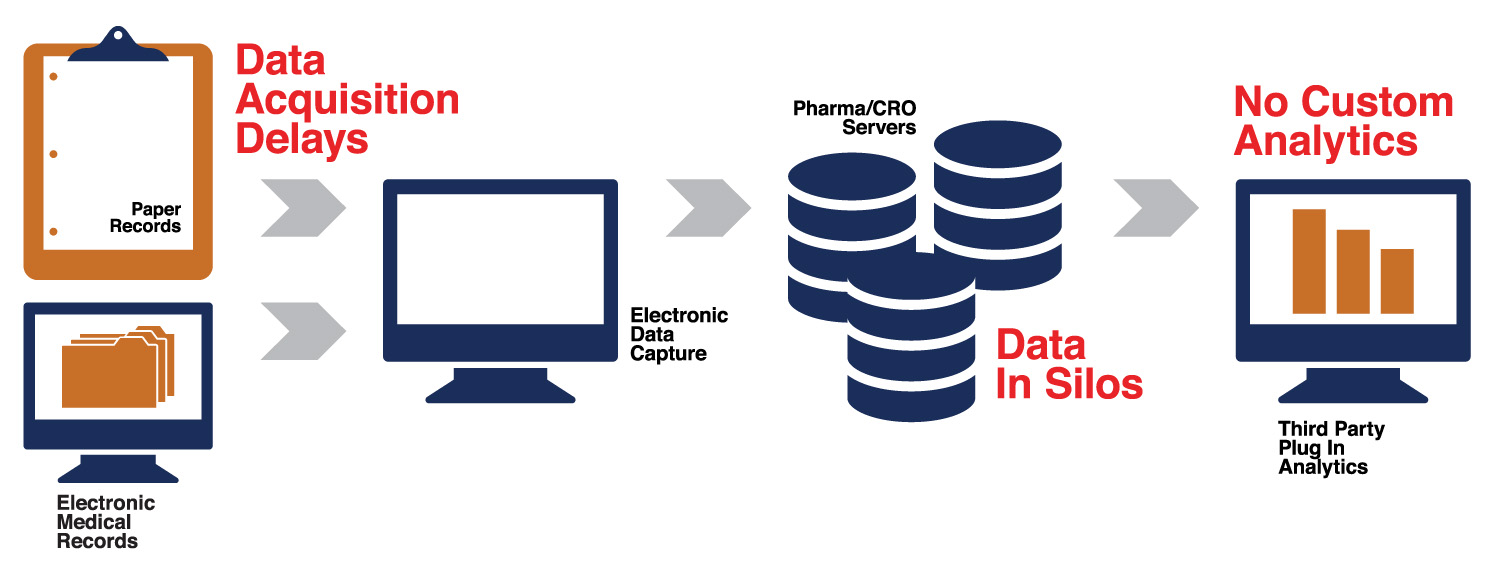
Extraordinary amounts of time and effort are spent on these trials, but the outcomes are dismal. Greater then $2 billion dollars is spent on developing a new drug. Even with these resources, the outcomes are uncertain and pharma's have to look at M&A and biotech acquisitions for growth.
Our product tackles these problems head on.
iClinical Solution
iClinical is a real-time data aggregation, analtyics and collaboration platform for clinical trials. We do this by collecting clinical trial data on Android tablets in protocol customized data aggregation and analytics apps. The data collection happens happens at source right in front of the subject (eSource). Any pateint reported outcomes is collected at this time, using the study tablet.
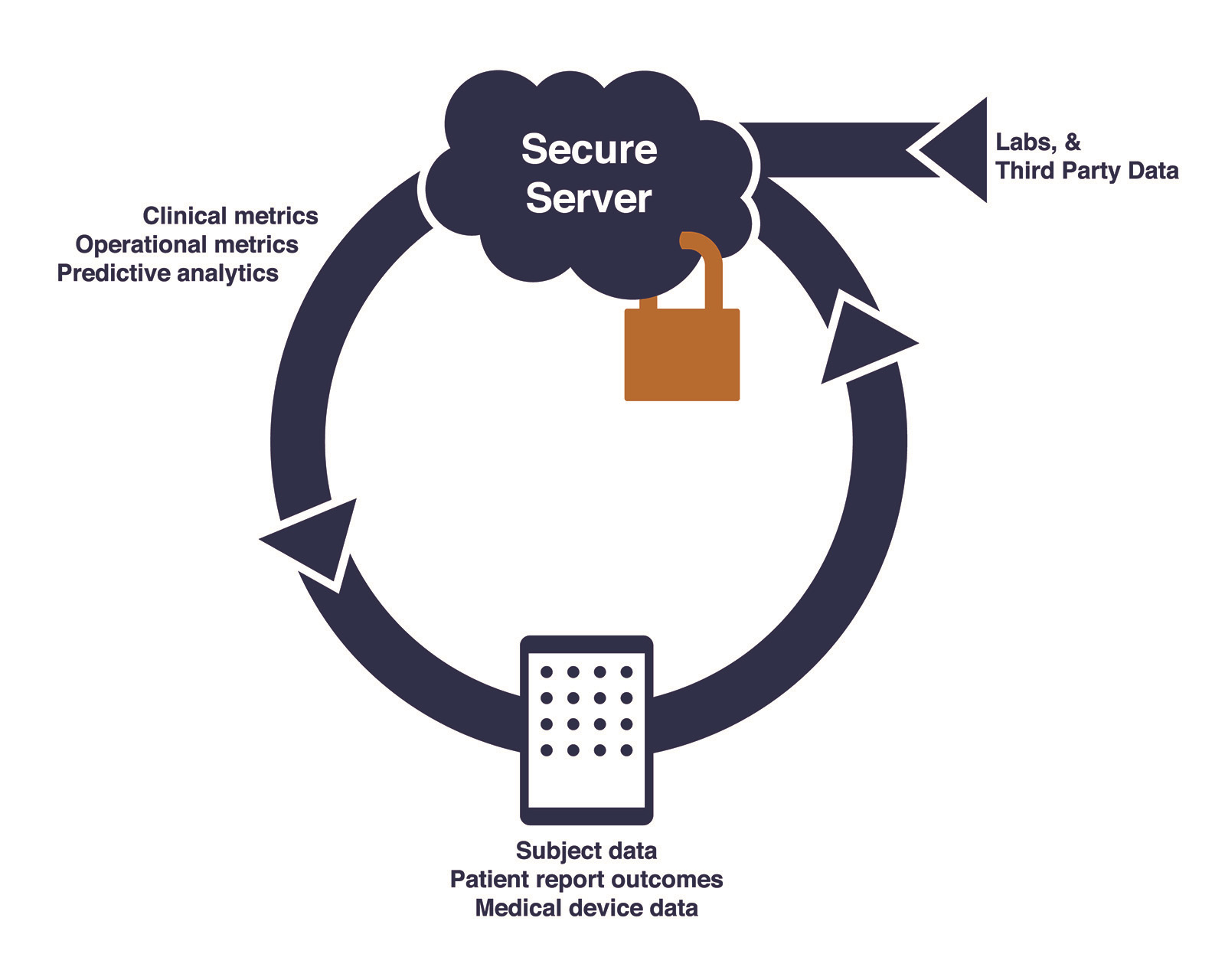
We then store all this data in a secure HIPAA compliant cloud server. While we are at it, we will also store lab data, patient reported outcomes and any other third party data on these servers.
Finally, the magic happens, the trial analytics are displayed in the analytics app on the study tablets. The analytics consists of all the trial metrics that the pharma company, trial monitors and the project physician want to see. These include summaries of clinical events (AEs, medication, trial end points), operational metrics (patient enrollment, data entry, queries and site performace) and predictive analytics (AE's compared to other trials, is the trial working?)
The Benefits
Quick trial start-up: We will get you up and running quickly - customized for the protocol and quickly deployed. A traditional deployment can take 6-9 months. We can accomplish the same process in as little as 2-4 months
Vertically integrated, end to end, out of the box solution: Data aggregation, storage and analytics is one seamless function on our platform. There is no need for separate vendors/systems to manage the whole system. That means less time and resources wasted configuring different solutions.
Built-in security: All the data from the Android tablet to secure cloud server is completely encrypted, the same happens once the analytics is fed back into the tablet. With an HIPAA compliant AWS server configuration, we can make sure all your data is one central secure safe location throughout the clinical trial and this is quickly accessible for real-time analytics.
Real time analytics on the tablet with zero programming: Pre-configured real time analytics means all the pre-programmed analytics dash boards and visual displays get populated as the data flows in. You will know up to the minute status of your clinical trial - the number of adverse events, serious adverse events, medications and all the trial end points are displayed right on the tablet. Since the analytics is programmed at the start of the trial, there is no need for programmers to extract the data and run reports. All the analtyics is available anytime/anywhere on the study tablet.
Actionable insights and trial metrics to stop non-working trials quickly: The principal investigator can now monitor the trial remotely and get actionable insights in real-time, he can take crucial decisions about the study in real-time. If the trial is not working he can make a quick decision to stop the study. Or if there are greater incidences of adverse events or trial end points, he can quickly change the course of the trial by making certain changes to the study. There is time and money saved by not prolonging those crucial study decisions.
Remote monitoring: With eSource and real time analytics, there is no need for frequent site visits by trial monitors. They can now monitor the trial remotely and issue queries/resolve trial issues remotely. This means there are massive cost savings both in number of monitors needed to monitor the trial and elimination of travel costs associated with such visits.
Real-time issue resolution: Real-time collaboration on a study tablet means time sensitive trial issues get resolved in real-time. Clinical trial monitors can now issue queries from the field and get immediate responses. Real-time chat over the platform means trial issues get resolved as and when they appear. Timely issue resolution means faster closure of study database and drug gets to the market sooner.
Pay per use solution: Our software is provided as a service (SAAS), which means you only pay for the trials that use our product and only for the duration for the trial. If for any reason you stop a clinical trial, you close out the trial and pay only for the duration that the trial was conducted. This also means there is no upfront licence fee payment for software that you might not fully use. We charge a one time study customization fee at the start of the trial and an ongoing SAAS fee based on number of subjects enrolled in the trial.
The Outcome
Drug gets to the market sooner and a lower cost: For a phase 3 clinical trial we can save 6-8 months of a 3-4 year trial. That means a few hundred million dollars in additional revenue. There is also few hundred million dollars in cost savings due to lower monitoring costs, data cleaning costs and fewer study personnel needed to run the study.
The Team
Sridhar Byrappa (CEO): Has 7+ years working in large pharmaceutical companies (Merck, Wyeth, Schering Plough). While working at these companies, he experienced first hand delays and cost overruns in drug development. iClinical was his response to elleivate some of these problems and bring life saving drugs to market sooner. He has a PhD in Microbiology/Immunology and an MBA in strategic management/International business.
Varsha Venkatesh (CTO): Is a data scientist with MS in Neuroscience from Max Planck Research Institute and Bachelors in Engineering. She has worked on several enterprise solutions involving cloud and big data. She is responsible for engineering a enterprise class product that has seamless data aggregation, analytics and collaboration tools built on a mobile/cloud platform.
Prachi Snehal (COO): Has 5+ years of consulting experience at Deloitte, solving complex technical and operational challenges at Fortune 500 companies. She is responsible for product (big data analytics/cloud) and business development. She has an MBA from Columbia Business School and Bachelors in Engineering.
Contact
We are currently running a 100 subject/20 site pilot clinical trial using our product. It is for a Glioblastoma drug. We would love to work on your trial. For a live demo or for more information, please feel free to call 848-248-0077 or email me at sridhar@iclinical.co